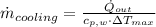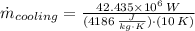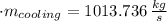Answer:
Step-by-step explanation:
The steam enters the condenser as a vapor-liquid mix and exits as a saturated liquid. Specific enthalpies at inlet and outlet are given from a property table for saturated water:
Inlet
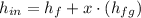
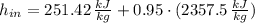
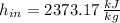
Outlet
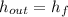
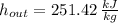
The heat transfer rate to the river is:
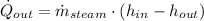
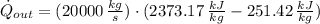
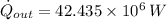
The mass flow rate of the cooling water is: