Answer:
5.36 grams the mass in grams of zinc nitrate the chemist has added to the flask.
Step-by-step explanation:
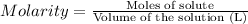
Moles of zinc nitrate = n
Volume of the solution = 135.0 mL = 0.1350 L
Molarity of the solution = 0.21 M
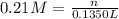
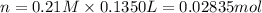
Mass of 0.02835 moles of zinc nitrate:
0.02835 mol × 189 g/mol = 5.358 g ≈ 5.36 g
5.36 grams the mass in grams of zinc nitrate the chemist has added to the flask.