Step-by-step explanation:
First, we will calculate the total charge as follows.
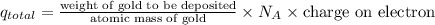
=
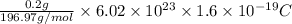
= 97.80 C
Now, we will calculate the current required by the jeweler to plate the bracelet as follows.
i =
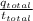
=
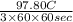
=
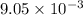 A
A
or, = 9.05 mA
thus, we can conclude that 9.05 mA current should he use to electroplate the bracelet in three hours.