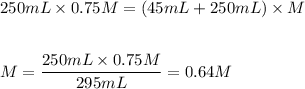Answer:

Step-by-step explanation:
When you form a diluted solution from a mother (concentrated) solution, the moles of solute are determined by the mother solution.
The main equation is:
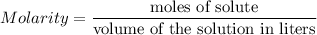
Then, since the moles of solute is the same for both the mother solution and the diluted solution:
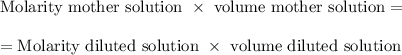
Substitute and solve for the molarity of the diluted solution: