Step-by-step explanation:
The given data is as follows.
Pressure (P) = 170 torr, mass of heptane (m) = 86.7 g
First, we will calculate the number of moles as follows.
No. of moles =
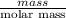
=
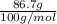
= 0.867 mol
Now, the number of moles of
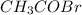 are calculated as follows.
are calculated as follows.
No. of moles =
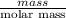
=
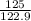
= 1.07
Therefore, mole fraction of heptane will be calculated as follows.
Mole fraction =
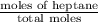
=
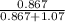
=
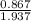
= 0.445
Now, we will calculate the partial pressure of heptane as follows.
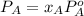
=
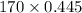
= 75.65 torr
Thus, we can conclude that the partial pressure of heptane vapor above this solution is 75.65 torr.