Answer:
I) Heat capacity of ice (Sensible, ice), II) Enthalpy of fusion of water (Latent, fusion) and III) Heat capacity of water (Sensible, water).
Step-by-step explanation:
At standard pressure, water has a fusion point and boiling point of 273.15 K and 373.15 K, respectively. Then, the total change in enthalpy is the sum of two sensible indicators and one latent indicator. That is:
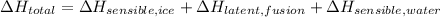
The needed physical constants are: Heat capacity of ice (Sensible, ice), Enthalpy of fusion of water (Latent, fusion) and Heat capacity of water (Sensible, water).