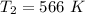Answer:
Therefore,
The final kelvin temperature when the volume of the gas is changed is
Step-by-step explanation:
Given:
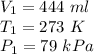
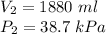
To Find:
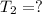
Solution:
Combined Gas Law:
The combined gas law combines the three gas laws:
Boyle's Law, Charles' Law, and Gay-Lussac's Law.
It states that the ratio of the product of pressure and volume and the absolute temperature of a gas is equal to a constant.
Hence,
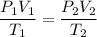
Substituting the values we get
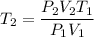
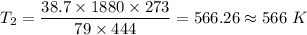
Therefore,
The final kelvin temperature when the volume of the gas is changed is