Answer:
E= 3.82 ×
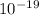 J
J
Step-by-step explanation:
The energy gap between the ground state and the excited state in the laser material is equal to the energy of single 521 nm photons emitted when a single electron relaxes from the excited to ground state, i.e.
E=hf
f = c / λ so
E = h c / λ
where,
h= plank constant= 6.626 ×
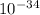 J s
J s
c = speed of light= 3.00 ×
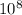 m/s
m/s
λ = wavelength of emitted photons= 521 ×
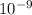 m
m
E = 6.626×
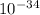 × 3.00×
× 3.00×
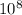 / 521×
/ 521×
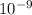
E= 3.82 ×
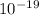 J
J