Answer :
(1)
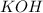 is act as a base and
is act as a base and
 act as an acid.
act as an acid.
(2) We can not classified as a base or an acid.
Explanation :
According to Arrhenius concept, a base is defined as a substance which donates hydroxide ions
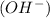 when dissolved in water and an acid is defined as a substance which donates hydronium ions
when dissolved in water and an acid is defined as a substance which donates hydronium ions
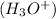 in water.
in water.
(1) The given chemical reaction is:
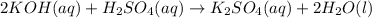
In this reaction,
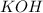 produces
produces
 ions and
ions and
 produces
produces
 ions.
ions.
Thus,
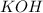 is act as a base and
is act as a base and
 act as an acid.
act as an acid.
(2) The given chemical reaction is:
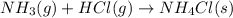
Arrhenius concept is only applicable on aqueous solution. Thus, we can not classified as a base or an acid.