Step-by-step explanation:
The given compounds are oxyacids and in these compounds more is the electronegativity of the central atom more will be its acidic strength.
This is because more is the electronegativity of the central atom more will be the polarity of OH bond. As a result, the compound can readily lose
 ion.
ion.
Also, more is the electronegativity of central atom more will be the stability of conjugate base formed.
Thus, we can conclude that given compounds are arranged in increasing acid strength as follows.
HOI <
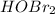 <
<
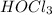 < HOF
< HOF