Answer:
 is the value of
is the value of
 for the acid HA.
for the acid HA.
Step-by-step explanation:
The pH of the solution = 3.75
The hydrogen ion concentration :
![pH=-log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/wyj0nahkywle04sx44478osqilvygxax2t.png)
![3.75=-\log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/gfqbmo25flp92rn2mzooryzqriwcak1c43.png)
![[H^+]=10^(-3.75)=0.0001778 M](https://img.qammunity.org/2021/formulas/chemistry/college/jojsdsdeb0vhzld9gupzzl875r5tsqpyx2.png)
Concentration of weak acid = 0.100 mole/L
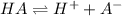
Initually
0.100 M 0 0
At equilibrium
(0.100-x)M x x
The expression of dissociation constant can be given as:
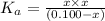
Here the value of x =
![[H^+]=0.0001778 M](https://img.qammunity.org/2021/formulas/chemistry/college/z0ufvouzuk07ce916xu1pdk6wco2enh9hb.png)
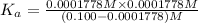
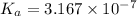
 is the value of
is the value of
 for the acid HA.
for the acid HA.