Answer: Volume of Freon = 12.23.
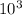 L
L
Step-by-step explanation: First, the temperatures have to be in the same unit, so all the tmeperatures is transformed into Kelvin:
T₁ = 192K
T₂ =
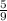 . (F - 32)+273.15
. (F - 32)+273.15
T₂ =
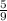 . (- 65 - 32)+273.15
. (- 65 - 32)+273.15
T₂ = 219.26K.
Second, BTU is an unit of energy. It can be transformed into Joules by the relation 1BTU = 1055.06J.
Q = 1055.06 . 46.9 = 49482.314J
The letter Q represents Heat and is calculated as Q = m.Cp.ΔT, where:
m is mass of the element;
Cp is the heat capacity specific for each element;
ΔT is the difference between the final and initial temperature;
Third, it will be needed the properties of the Freon:
Freon (CF2Cl2) = 120.91g/mol; Cp = 74J/mol.K; ρ = 1.49kg/m³
Fourth, we calculate the mass of Freon necessary for the remove of 46.9BTU of energy from the system:
Q = m.Cp.ΔT
m=
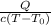
m =
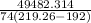
m = 18228.214g or m=18.228Kg
Fifth, using the density of Freon, Volume can be found
ρ =
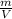
V = m / ρ
V = 18.228 / 1.49
V = 12.23m³
As SI for density is Kg/m³, the volume found is in m³.
Cubic meters is related to Liters as the following: 1m³=1000l.
So, volume of Freon = 12.23.
 L
L
The volume of Freon needed is 12.23.
 L.
L.