Answer: The number of carbon, hydrogen and oxygen atoms on the left side of the reaction are 12, 28 and 38 respectively
Step-by-step explanation:
In a chemical equation, the chemical species are termed as reactants or products.
Reactants are defined as the species which react in the reaction and are written on the left side of the reaction arrow.
Products are defined as the species which are produced in the reaction and are written on the right side of the reaction arrow.
For the given chemical equation:
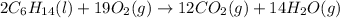
On the reactant side:
Number of carbon atoms = (6 × 2) = 12
Number of hydrogen atoms = (14 × 2) = 28
Number of oxygen atoms = (2 × 19) = 38
Hence, the number of carbon, hydrogen and oxygen atoms on the left side of the reaction are 12, 28 and 38 respectively