Answer: The reaction proceeds in the forward direction
Step-by-step explanation:
For the given chemical equation:
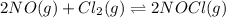
Relation of
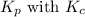 is given by the formula:
is given by the formula:
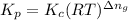
where,
 = equilibrium constant in terms of partial pressure = ?
= equilibrium constant in terms of partial pressure = ?
 = equilibrium constant in terms of concentration =
= equilibrium constant in terms of concentration =

R = Gas constant =
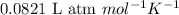
T = temperature =
![35^oC=[35+273]K=308K](https://img.qammunity.org/2021/formulas/chemistry/high-school/m5wyt46w64rj1fs32tvl18j2urmq0i9iaz.png)
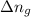 = change in number of moles of gas particles =
= change in number of moles of gas particles =
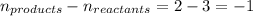
Putting values in above equation, we get:
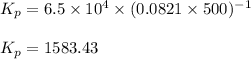
 is the constant of a certain reaction at equilibrium while
is the constant of a certain reaction at equilibrium while
 is the quotient of activities of products and reactants at any stage other than equilibrium of a reaction.
is the quotient of activities of products and reactants at any stage other than equilibrium of a reaction.
The expression of
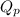 for above equation follows:
for above equation follows:
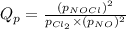
We are given:
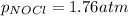
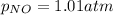
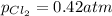
Putting values in above equation, we get:
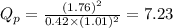
We are given:
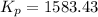
There are 3 conditions:
- When
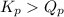 ; the reaction is product favored.
; the reaction is product favored. - When
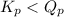 ; the reaction is reactant favored.
; the reaction is reactant favored. - When
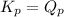 ; the reaction is in equilibrium
; the reaction is in equilibrium
As,
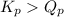 , the reaction will be favoring product side.
, the reaction will be favoring product side.
Hence, the reaction proceeds in the forward direction