Answer:
91383 J
Step-by-step explanation:
The equation of the reaction can be represented as:
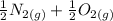 ------>
------>
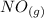
Given that:
The standard enthalpy of formation of NO(g) is 91.3 kJ⋅mol−1 at 298.15 K.
The equation below shown the reaction between the enthalpy of reaction at a particular temperature to another.
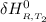 =
=
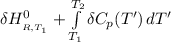
where:
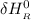 = enthalpy of reaction
= enthalpy of reaction
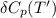 = the difference in the heat capacities of the products and the reactants.
= the difference in the heat capacities of the products and the reactants.
∴
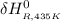 =
=
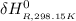

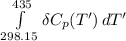
=
![1(91300 J.mol^(-1) ) +\int\limits^(435)_(298.15) [{(29.86)-(1)/(2)(29.38)-(1)/(2)29.13}]J.K^(-1).mol^(-1) \, dT'](https://img.qammunity.org/2021/formulas/chemistry/college/p3a2yl6kj5hdbawhcyfykaf37hpnc1x7dc.png)
= 91300 J + (0.605 J.K⁻¹)(435-298.15)K
= 91382.79 J
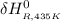 ≅ 91383 J
≅ 91383 J