Answer:
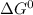 at 298 K is -101.0 kJ/mol
at 298 K is -101.0 kJ/mol
Step-by-step explanation:
According to thermodynamic of state,
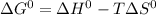
where,
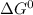 ,
,
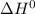 , T and
, T and
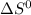 represent change in free energy in standard state, change in enthalpy in standard state, temperature in kelvin scale and change in entropy in standard state.
represent change in free energy in standard state, change in enthalpy in standard state, temperature in kelvin scale and change in entropy in standard state.
Here,
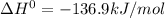 , T = 298 K and
, T = 298 K and
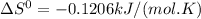
So,
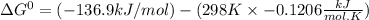
= -101.0 kJ/mol
So,
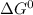 at 298 K is -101.0 kJ/mol
at 298 K is -101.0 kJ/mol