Answer:
[CH₃NH₃⁺] = 0.00355 M
[CH₃NH₂] = 0.028 M
pH = 11.55
Step-by-step explanation:
The reaction is the following:
CH₃NH₂ + H₂O ⇄ CH₃NH₃⁺ + OH⁻ (1)
0.0317 - x x x
The equilibrium constant of the reaction (1) is:
![K_(b) = ([CH_(3)NH_(3)^(+)][OH^(-)])/([CH_(3)NH_(2)])](https://img.qammunity.org/2021/formulas/chemistry/college/axrv31kfo4lkyktawqajr71rwcue53ov8h.png)
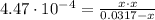
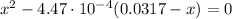 (2)
(2)
By solving equation (2) for x, we have:
x₁ = -0.00399
x₂ = 0.00355
Taking the positive value, we have that:
x = [CH₃NH₃⁺] = [OH⁻] = 0.00355 M
[CH₃NH₂] = 0.0317 - x = (0.0317 - 0.00355)M = 0.028 M
Therefore, the concentrations of CH₃NH₂ and CH₃NH₃⁺ are 0.028 M and 0.00355 M, respectively.
The pH of the solution is:
![pOH = -log [OH^(-)] = -log (0.00355) = 2.45](https://img.qammunity.org/2021/formulas/chemistry/college/isf5q7mrb1nxmeye3iqka7knutdpi1hxwe.png)
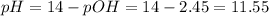
Hence, the pH of the solution is 11.55.
I hope it helps you!