Answer:
101.2%
Step-by-step explanation:
Given:
Theoretical yield of the precipitate = 0.914 g
Actual yield of the precipitate = 0.925 g
Now, the percent yield is given as a ratio of actual yield by theoretical yield expressed as a percentage.
Framing in equation form, we have:
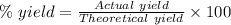
Now, plug in 0.925 g for actual yield, 0.914 g for theoretical yield and solve for % yield. This gives,
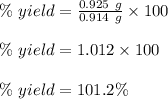
Therefore, the percent yield is 101.2%.