Answer:
a) 18.81% is the mass percent (m/m) of the KCl solution.
b) 3.643 M is the molarity of the KCl solution.
c) 0.6073 M is the molarity of the diluted KCl solution.
Step-by-step explanation:
a) mass on of evaporating dish = 24.10 g = x
mass on of evaporating dish and KCl solution = 44.30 g = y
After heating, mass on of evaporating dish and dry KCl = 27.90 g = z
Mass of KCl solution = y - x = 44.30 g - 24.10 g = 20.2 g
Mass of KCl in solution = z - x = 27.90 g - 24.10 g = 3.8 g
The mass percent (m/m) of the KCl solution;
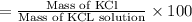
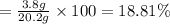
18.81% is the mass percent (m/m) of the KCl solution.
b) Moles of KCl =

Volume of the KCl solution = 14.0 mL = 0.014 L ( 1 mL = 0.001 L)
Molarity of the solution :
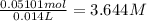
3.643 M is the molarity of the KCl solution.
c)
Molarity of KCl solution before dilution =
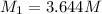
Volume of the solution before dilution =
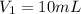
Molarity of KCl solution after dilution =
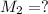
Volume of the solution after dilution =
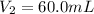

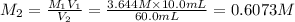
0.6073 M is the molarity of the diluted KCl solution.