Answer:
The compounds in order from largest mass percent of nitrogen to smallest mass percent of nitrogen:
Urea > Ammonium sulfate > ammonium hydrogen sulfate
Step-by-step explanation:
Percentage of mass of an element in a compound :
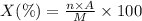
Where:
n = Number of atoms X
A = Atomic mass of atom X
M = Molar mass of compound
Percentage of nitrogen in urea;
Molar mass of urea,M = 60 g/mol
Atomic mass of nitrogen, A = 14 g/mol
n = 2
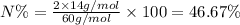
Percentage of nitrogen in ammonium sulfate ;
Molar mass of urea,ammonium sulfate = 132 g/mol
Atomic mass of nitrogen, A = 14 g/mol
n = 2
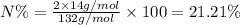
Percentage of nitrogen in ammonium hydrogen sulfate;
Molar mass of ammonium hydrogen sulfate ,M = 115 g/mol
Atomic mass of nitrogen, A = 14 g/mol
n = 2
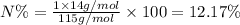
The compounds in order from largest mass percent of nitrogen to smallest mass percent of nitrogen:
46.67% > 21.21%>12.17%
Urea > Ammonium sulfate > ammonium hydrogen sulfate