Answer:
2.8 g/mL is the density of the mineral.
Step-by-step explanation:
The mass,
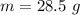
Volume of the object = Volume of water level rose - Initial volume of water = 30.5 mL - 20.3 mL = 10.2 mL
The expression for the calculation of density is shown below as:-
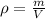
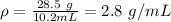
2.8 g/mL is the density of the mineral.