Answer:
3.49 Liters of hydrogen is produced when 2.80 grams of aluminum reacts at STP.
Step-by-step explanation:
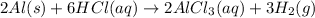
Moles of aluminum =
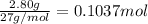
According to reaction , 2 moles of aluminium gives 3 moles of hydrogen gas.Then 0.1037 moles of aluminum will give:
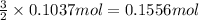 of hydrogen gas
of hydrogen gas
Moles of hydrogen gas = n = 0.1556 mol
Pressure of the gas, at STP , P = 1 atm
Temperature of the gas, at STP = T = 273 K
Volume of the gas , At STP = V
 ( Ideal gas equation )
( Ideal gas equation )
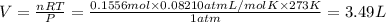
3.49 Liters of hydrogen is produced when 2.80 grams of aluminum reacts at STP.