Answer: The mass of BHT that can be formed is 18.8 grams
Step-by-step explanation:
To calculate the number of moles, we use the equation:
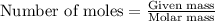 .....(1)
.....(1)
Given mass of t-butanol =
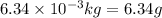 (Conversion factor: 1 kg = 1000 g)
(Conversion factor: 1 kg = 1000 g)
Molar mass of t-butanol = 74 g/mol
Putting values in equation 1, we get:
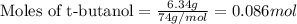
The chemical equation for the formation of BHT from t-butanol follows:
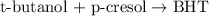
By Stoichiometry of the reaction:
1 mole of t-butanol produces 1 mole of BHT
So, 0.086 moles of t-butanol will produce =
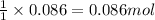 of BHT
of BHT
Now, calculating the mass of BHT from equation 1, we get:
Molar mass of BHT = 220 g/mol
Moles of BHT = 0.086 moles
Putting values in equation 1, we get:
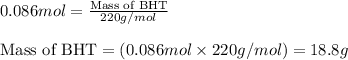
Hence, the mass of BHT that can be formed is 18.8 grams