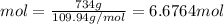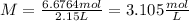Answer:
Concentration = 3.105 mol/L
Step-by-step explanation:
To determine the concentration of a solution it can be identified by means of the Molarity equation:
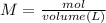
Milliliters to liters are converted:
2510 mL = 2.150 L
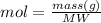
The molecular weight of Li2SO4 is determined for the calculation of moles: