Answer:
The order of the reaction is 1.
Step-by-step explanation:
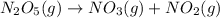
The rate law of the reaction ;
![R=k[N_2O_5]^x](https://img.qammunity.org/2021/formulas/chemistry/college/ujv2rl788b5vcteryuqehntx345n3k78os.png)
The rate of the reaction from T = 0 s to 25 s,
![[N_2O_5]=1.000 M](https://img.qammunity.org/2021/formulas/chemistry/college/8qkrwbfklaykcq10zqd4adpjomufhrvs0g.png) to
to
![[N_2O_5]=0.822 M](https://img.qammunity.org/2021/formulas/chemistry/college/av99qlxjeovu13gko3s9oxp2dgtzxnlhs5.png) respectively.
respectively.
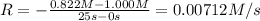
![0.00712 M/s=k[0.822 M]^x](https://img.qammunity.org/2021/formulas/chemistry/college/piz1yqcc4om8v8syscudj6erv1gxvdimyz.png) ..[1]
..[1]
The rate of the reaction from T = 25 s to 50 s,
![[N_2O_5]=0.822 M](https://img.qammunity.org/2021/formulas/chemistry/college/av99qlxjeovu13gko3s9oxp2dgtzxnlhs5.png) to
to
![[N_2O_5]=0.677 M](https://img.qammunity.org/2021/formulas/chemistry/college/3hjryhww7rajnqjpqk9ucy6h4ieqv97x5d.png) respectively.
respectively.
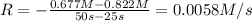
![0.00646 M/s=k[0.677 M]^x](https://img.qammunity.org/2021/formulas/chemistry/college/lhbk2n3ptr2adquwdq7utuc50qw2dliyhz.png) ..[2]
..[2]
[1] ÷ [2]
![(0.00712 M/s)/(0.0058 M/s)=(k[0.822 M]^x)/(k[0.677 M]^x)](https://img.qammunity.org/2021/formulas/chemistry/college/3s678hi0k67anovrte8n2jwjgurm6wav4c.png)
Solving for x:
x = 1.05 ≈ 1
The rate law of the reaction ;
![R=k[N_2O_5]^1](https://img.qammunity.org/2021/formulas/chemistry/college/354d3cuo63ysaybytuvjzoah3dkz5btn80.png)
The order of the reaction is 1.