The question is incomplete, here is the complete question:
A chemistry student needs of 60.0 g of 4-methyl-2-pentanone for an experiment. He has available 350. g of a 14.0 % w/w solution of 4-methyl-2-pentanone in diethyl ether. Calculate the mass of solution the student should use. If there's not enough solution, press "No solution" button
Answer: There is not enough solution.
Step-by-step explanation:
We are given:
Mass of 4-methyl-2-pentanone needed = 60.0 gram
14 % w/w 4-methyl-2-pentanone solution
This means that 14 grams of 4-methyl-2-pentanone is present in 100 grams of solution
Applying unitary method:
If 14 grams of 4-methyl-2-pentanone is present in 100 grams of solution
So, 60 grams of 4-methyl-2-pentanone will be present in =
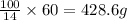
As, the given amount of solution is less than the calculated amount.
Hence, there is not enough solution.