Step-by-step explanation:
It is given that pH is equal to 9.2. So, we will calculate the value of
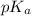 as follows.
as follows.
pH =
![pK_(a) + log \frac{[H_(3)O^(+)]}{[\text{solution}]}](https://img.qammunity.org/2021/formulas/chemistry/college/2yryu9f4l5u0vslzdxw681gij4fb0vnjxl.png)
Putting the given values into the above formula as follows.
pH =
![pK_(a) + log \frac{[H_(3)O^(+)]}{[\text{solution}]}](https://img.qammunity.org/2021/formulas/chemistry/college/2yryu9f4l5u0vslzdxw681gij4fb0vnjxl.png)
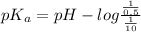
= 7.9
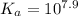
=
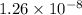
Since, the value of
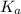 comes out to be positive. This means that there will occur an increase in the value of
comes out to be positive. This means that there will occur an increase in the value of
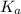 or
or
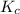 .
.