Answer:
5.93 x 10²³atoms
Step-by-step explanation:
The chemical formula of the compound Aspartame is:
C₁₄H₁₈N₂O₅
Unknown:
Number of atoms = ?
To solve for this problem, we need to find the mass of carbon that makes up the compound Aspartame;
Molar mass of C₁₄H₁₈N₂O₅ = 14(12)+ 18(1)+ 2(14)+ 5(16 ) = 294g/mol
Find the number of moles of this mass:
Number of moles =
 =
=
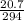 = 0.07moles
= 0.07moles
1 mole of Aspartame contains 14 moles of C
0.07 moles of Aspartame will contain 14 x 0.07 = 0.99moles of C
To find the number of atoms;
Number of atoms = number of moles x 6.02 x 10²³
1 mole of a substance = 6.02 x 10²³ atoms;
0.99moles of C = 5.93 x 10²³atoms