Answer: The equilibrium constant,
 for the given reaction is 6.653
for the given reaction is 6.653
Step-by-step explanation:
We are given:
Initial partial pressure of nitrogen dioxide = 1.00 atm
Initial partial pressure of dinitrogen tetraoxide = 1.500 atm
Equilibrium partial pressure of nitrogen dioxide = 0.512 atm
For the given chemical equation:
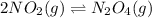
Initial: 1.00 1.500
At eqllm: 1.00-2x 1.500+x
Evaluating the value of 'x'
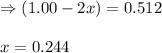
So, equilibrium partial pressure of dinitrogen tetraoxide = (1.500 + x) = [1.500 + 0.244] = 1.744 M
The expression of
 for above equation follows:
for above equation follows:
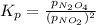
Putting values in above equation, we get:
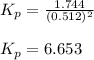
Hence, the equilibrium constant,
 for the given reaction is 6.653
for the given reaction is 6.653