The question is incomplete. The complete question is:
An analytical chemist weighs out 0.093g of an unknown monoprotic acid into a 250mL volumetric flask and dilutes to the mark with distilled water. He then titrates this solution with 0.1600M NaOH solution. When the titration reaches the equivalence point, the chemist finds he has added 6.5mL of NaOH solution. Calculate the molar mass of the unknown acid. Round your answer to 2 significant digits.
Answer: The molar mass of the unknown acid is 89 g/mol
Step-by-step explanation:
To calculate the molarity of solution, we use the equation:
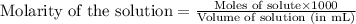
Molarity of NaOH solution = 0.1600 M
Volume of NaOH solution = 6.5 mL
Putting values in above equation, we get:
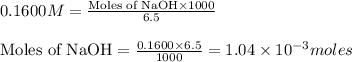
The chemical equation for the reaction of monoprotic acid and NaOH follows:

By Stoichiometry of the reaction:
1 mole of NaOH reacts with 1 mole of HA
So, moles of NaOH will react with =
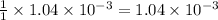 moles of HA
moles of HA
To calculate the molar mass for given number of moles, we use the equation:

Given mass of HA = 0.093 g
Moles of monoprotic acid =
Putting values in above equation, we get:
