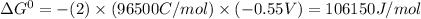Answer:
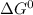 for the given reaction is 106150 J/mol
for the given reaction is 106150 J/mol
Step-by-step explanation:
Oxidation:
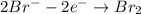
Reduction:

------------------------------------------------
Overall:
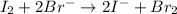
We know,
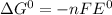
where, n is number of electron exchanged during overall reaction and 1 F equals to 96500 C/mol
Here, n = 2 and
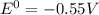
So,