Answer:
pOH of resulting solution is 0.086
Step-by-step explanation:
KOH and CsOH are monoacidic strong base
Number of moles of
 in 375 mL of 0.88 M of KOH =
in 375 mL of 0.88 M of KOH =
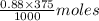 = 0.33 moles
= 0.33 moles
Number of moles of
 in 496 mL of 0.76 M of CsOH =
in 496 mL of 0.76 M of CsOH =
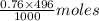 = 0.38 moles
= 0.38 moles
Total volume of mixture = (375 + 496) mL = 871 mL
Total number of moles of
 in mixture = (0.33 + 0.38) moles = 0.71 moles
in mixture = (0.33 + 0.38) moles = 0.71 moles
So, concentration of
 in mixture,
in mixture,
![[OH^(-)]](https://img.qammunity.org/2021/formulas/chemistry/college/7idhlmby70nqtuzatxrjo3z5yjkhl79yjb.png) =
=
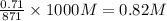
Hence,
![pOH=-log[OH^(-)]=-log(0.82)=0.086](https://img.qammunity.org/2021/formulas/chemistry/college/vlo29e9kpohdtniz597t7chkhxobt0dlbb.png)