The question is incomplete, here is the complete question:
0.100 g of an unknown protein are dissolved in enough solvent to make 5.00mL of solution. The osmotic pressure of this solution is measured to be 0.0604atm at 25.0°C. Calculate the molar mass of the protein. Be sure your answer has the correct number of significant digits.
Answer: The molar mass of the protein is 8097.2 g/mol
Step-by-step explanation:
To calculate the concentration of solute, we use the equation for osmotic pressure, which is:
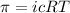
where,
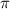 = osmotic pressure of the solution = 0.0604 atm
= osmotic pressure of the solution = 0.0604 atm
i = Van't hoff factor = 1 (for non-electrolytes)
c = molarity of solute = ?
R = Gas constant =
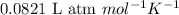
T = temperature of the solution =
![25^oC=[273+25]=298K](https://img.qammunity.org/2021/formulas/chemistry/college/5ofe7r0qj6k85y62ayk6z4ogxpmpululvn.png)
Putting values in above equation, we get:
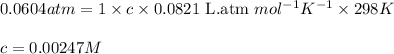
To calculate the molecular mass of solute, we use the equation used to calculate the molarity of solution:
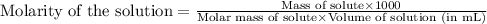
We are given:
Molarity of solution = 0.00247 M
Given mass of protein = 0.100 g
Volume of solution = 5.00 mL
Putting values in above equation, we get:
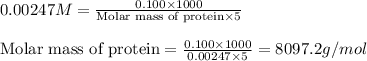
Hence, the molar mass of the protein is 8097.2 g/mol