2. 0.05544 moles of hydrogen atom are present in 154 mL 0.18 M solution of H2SO4.
3. 15.2506 heat in Joules is absorbed by 150.0 mL of pure water that is heated from 21.2°C to 45.5°C.
4. 0.75 M is the concentration of Na+ ions in 25.0 mL of 1.50 M NaOH is reacted with 25.0 mL of 1.50 M HCI
Step-by-step explanation:
Number of moles of H2SO4 can be calculated by the given volume and molarity from the formula:
molarity =
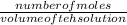
number of moles = molarity × volume of the solution of H2SO4
number of moles = 0.18 × 0.154 litres
= 0.02772 moles of H2SO4.
Since 1 mole of H2SO4 contains 2 moles of hydrogen
so, 0.02772 moles of H2SO4 will have x moles
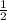 =
=
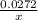
2 × 0.02772 = x
0.05544 moles of hydrogen atom are present in 154 mL 0.18 M solution of H2SO4.
3. The heat absorbed is calculated from the formula:
ΔH = cp × m × ΔT ( ΔT = change in temperature in Kelvin, m in kg, cp= specific heat of water)
putting the values in formula:
ΔH = 4.184 × 0.15 × ( 318.65-294.35)
= 4.184 × 0.15× 24.3
= 15.2506 Joules of heat is absorbed.
4. The concentration of Na+ ions
the balanced equation is
NaOH + HCl⇒ NaCl + H20
from one mole of NaCl 1 mole of NaCl i.e one 1 mole of Na+ ions is formed.
number of moles of NaOH is calculated by the formula:
Molarity =
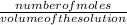
number of moles = 0.025L × 1.50M
= 0.0375 moles of NaOH
so 1 mole of NaOH produces 1 mole of Na= ions
hence, 0.0375 moles produces x moles of Na+ ions
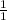 =
=
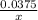
moles of Na+ is produced.
concentration of NaOH in 50 ml solution because NaOH and HCl of 25 ml reacted.
applying the molarity formula from above
Molarity =
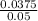
= 0.75 M