2.488 grams of MgO is produced when 2.49 grams of Mg reacts with excess of oxygen.
Step-by-step explanation:
The balanced equation for magnesium oxide produced is given by:
2Mg +
 ⇒ 2MgO
⇒ 2MgO
it can be seen from the equation that 2 moles of Mg will produce 2 moles of MgO
from the mass of the MgO given moles of Mg can be calculated, the atomic mass of Mg is 24 gm/mole
the formula to calculate number of moles:
number of moles (n) =
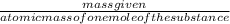
n=
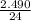
n = 0.1037 moles of Mg reacts with excess of oxygen.
Applying stoichiometry,
2 moles of Mg yields 2 mole of MgO
0.1037 moles of Mg yields x mole of MgO
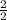 =
=
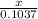
2x = 2 × 0.1037
x = 0.1037 moles of MgO is formed.
The mass of MgO produced could be known by multiplying atomic weight with number of moles.
mass = 0.1037 × 24
= 2.488 grams of MgO wiil be produced.