Answer:
Step-by-step explanation:
Percent dissociation is the amount of the substance dissociated per 100 parts.
Determine how much dissociate from a 0.55 M solution.
1. Write the dissociation equation:
C₃H₅O₃H ⇄ C₃H₅O₃H⁻ + H⁺
2. Build the ICE (initial, change, equilibrium) table:
C₃H₅O₃H ⇄ C₃H₅O₃H⁻ + H⁺
I 0.55M 0 0
C - x +x +x
E 0.55 - x x x
3. Write the equilibrium equation and calculate x
- 1.4 × 10⁻⁴ = x² / (0.55 -x)
- x² + 0.00014x - 0.000077 = 0
Use the quadratic equation:
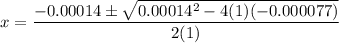
The positve value is x = 0.0087
4. Calculate the percent dissociation, α