2.1618 grams of P4 is required to produce 4.21x10^22 molecules of phosphorus trifluoride.
Step-by-step explanation:
The balanced chemical reaction for formation of PF3 from yellow phosphorus is given by:
P4 + 6F2 ⇒ 4PF3
1 mole of P4 reacts to give 4 moles of PF3
It is given that 4.21x10^22 molecules of PF3 are produced
so the number of moles can be calculated by the relation
number of molecules = number of moles × Avagadro number
number of moles =
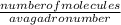
n =
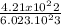
n= 0.06989 moles of PF3 is formed.
Applying stoichiometry,
1 mole of P4 gives 4 moles PF3
x mole will produce 0.06989 moles of PF3
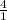 =
=
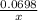
4x= 0.0698 × 1
x =
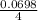
x= 0.01745 moles of P4 will be required.
The weight of the phosphorus can be obtained by number of moles × atomic mass of one mole of P4
= 0.01745 × 123.89
= 2.1618 grams of P4.