Answer:
The half life of the substance is
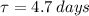 .
.
Explanation:
The equation that models the amount of substance after time
 is
is
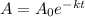 .
.
We are told that that the initial amount
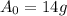 , and the k-value is
, and the k-value is
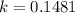 ; therefore,
; therefore,
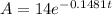
The half-life of the substance is the amount of time
 it takes to decay to half its initial value; therefore,
it takes to decay to half its initial value; therefore,
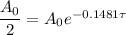
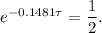
Take the Natural Logarithm of both sides and get:
![ln[e^(-0.1481\tau ) ]= ln[(1)/(2)]](https://img.qammunity.org/2021/formulas/mathematics/middle-school/zww3xkv2x0dqxk3dvmenckvggei6wni57e.png)
![-0.1481\tau = ln[(1)/(2) ]](https://img.qammunity.org/2021/formulas/mathematics/middle-school/hxrfalyir9g8ypnn6op8g3sy88bcgz0i3m.png)
![\tau = (ln[(1)/(2) ])/(-0.1481)](https://img.qammunity.org/2021/formulas/mathematics/middle-school/77595461i0qndjxwonqgw4qdkxehhevzth.png)
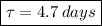
Thus, we find that the half life of the substance is 4.7 days.