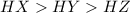Answer:
Decreasing order of strength of the the acids :
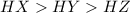
Step-by-step explanation:
The strength of an acid is measured by their pH of aqueous solution.
![pH=-\log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/fi7xbn2q6p6sosuqayohrecmxrbau6j4s5.png)
![pH\propto (1)/([H^+])](https://img.qammunity.org/2021/formulas/chemistry/college/dvo96b57c9f02w6646blg539n3kestcyaz.png)
- Lower the pH more will be the hydrogen ions and stronger will be the acid.
- Higher the pH less will be the hydrogen ions and weaker will be the acid.
Solution of HX , has equal number of hydrogen ions as a that of its initial molecules o HX.
![[H^+]_x=[HX]](https://img.qammunity.org/2021/formulas/chemistry/college/gur83l1kcbyj9w7sv59gyxncg1vndv4cdw.png)
Solution of HY , molecules of hydrogen ions are half of the molecules of HY.
![[H^+]_y=(1)/(2)[HY]](https://img.qammunity.org/2021/formulas/chemistry/college/saiypqog4vwk5a212tt9v39b5fqpukhirj.png)
Solution of HZ , one quarter molecules of hydrogen ions and three quarter of the molecules of HY.
![[H^+]_z=(1)/(4)[HZ]](https://img.qammunity.org/2021/formulas/chemistry/college/6009s2zmrsxuuopll0t3uo3421xy7v0br9.png)
![[H^+]_x>[H^+]_y>[H^+]_z](https://img.qammunity.org/2021/formulas/chemistry/college/bqswngyeubhi5m9vsa3wriei1f13zeufez.png)
This means that HX is strongest acid followed by HY and then HZ.
Decreasing order of strength of the the acids :