Answer:
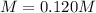
Step-by-step explanation:
Hello,
In this case, the undergone chemical reaction is:
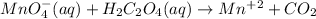
In such a way, the acidic redox balance turns out:
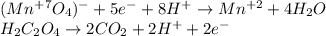
Which leads to the total balanced equation as follows:
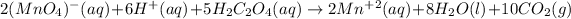
Thus, as the mass of oxalic acid is not given, one could suppose a value of 1 g (which you can modify based on the actual statement) in order to compute the oxalic acid moles as shwon below:
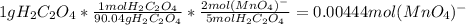
Whereby the molality results:
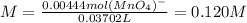
Remember you can modify the oxalic acid mass as you desire.
Best regards.