Answer:
the ratio of the kinetic energy of an electron to that of a proton if their wavelengths are equal is 1835.16 .
Step-by-step explanation:
We know, wavelength is expressed in terms of Kinetic Energy by :
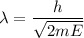
Therefore ,
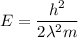
It is given that both electron and proton have same wavelength.
Therefore,
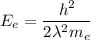 .... equation 1.
.... equation 1.
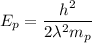 .... equation 2.
.... equation 2.
Now, dividing equation 1 by 2 .
We get ,
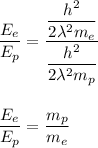
Putting value of mass of electron =
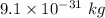 and mass of proton =
and mass of proton =
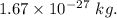
We get :
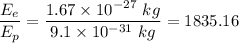
Hence , this is the required solution.