Answer:
The rate of reaction :
![R* 3=-(d[O_2])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/college/e1meputm50zgx69rnu8ibg4apopch8jfth.png)
![R* 2=(d[O_3])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/college/icdzed2rlgvve9xn5c19w5iql2up0je9gq.png)
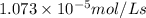 is the rate of appearance of an ozone.
is the rate of appearance of an ozone.
Step-by-step explanation:
The rate of the reaction is defined as change in in the concentration of any one of the reactants or products per unit time.
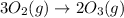
a) Rate of the reaction = R
![R=(-1)/(3)(d[O_2])/(dt)=(1)/(2)(d[O_3])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/college/kgcsxredn3rfnyoa6ik1dc4j7kv0lj6pls.png)
Rate of the disappearance of the oxygen gas:
![-(d[O_2])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/college/8sj3842lr9ag0tssfyk28cx2nx6lz9a9eo.png)
![R*3=-(d[O_2])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/college/jto6tj21ogydoclssk9gkukxy8984vkvf0.png)
Rate of the appearance of the ozone gas:
![(d[O_3])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/college/e48vye2vdvnfvlrtogizwackaduo6nrbd5.png)
![R* 2=(d[O_3])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/college/icdzed2rlgvve9xn5c19w5iql2up0je9gq.png)
b)Rate of the disappearance of the oxygen gas:
![-(d[O_2])/(dt)=1.61* 10^(-5) mol/L s](https://img.qammunity.org/2021/formulas/chemistry/college/dqm7xodayky8mjg0zl1c0beqe13lytnyuh.png)
![R*3=-(d[O_2])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/college/jto6tj21ogydoclssk9gkukxy8984vkvf0.png)
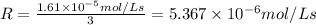
Rate of the appearance of the ozone gas:
![(d[O_3])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/college/e48vye2vdvnfvlrtogizwackaduo6nrbd5.png)
![R* 2=(d[O_3])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/college/icdzed2rlgvve9xn5c19w5iql2up0je9gq.png)
![5.367* 10^(-6) mol/L s* 2=(d[O_3])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/college/ko4kxxpn4agl2pgmnmsn286s84xl3guz7w.png)
![(d[O_3])/(dt)=1.073* 10^(-5) mol/Ls](https://img.qammunity.org/2021/formulas/chemistry/college/k7aq2d05rc27k8rsstxgrtngrz76dv2yk6.png)
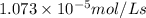 is the rate of appearance of an ozone.
is the rate of appearance of an ozone.