Answer:
The molar heat capacity of C₂H₆O = 2.797 J/g·°C
Step-by-step explanation:
The known parameters are first listed as follows from reading the question
Heat transferred, H = 1328 J
Mass of the heated ethanol, m = 40.4 g
Increase in temperature of the heated gas = ΔT = 13.4 °C
Molar mass of ethanol = 46.07 g/mol
Number of moles of ethanol = 40.4/46.07 = 0.877 moles
The heat required to raise the temperature of 40.4 g of ethanol by 13.4 °C is 1328 J
Therefore we have H = m·c·ΔT
c = specif heat capacity = H ÷ (m·ΔT) =
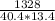 = 2.453 J/g·°C
= 2.453 J/g·°C
Therefore the heat required to raise the temperature of one mole of ethanol by one degree C =
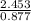 J/g·°C = 2.797 J/g·°C
J/g·°C = 2.797 J/g·°C
=