Answer : The final volume of the gas is, 34.8 L
Explanation :
Formula used :
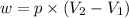
where,
w = work done = 137.1 J = 35.8 L.atm (1 L.atm = 101.3 J)
p = external pressure = 783 torr = 1.03 atm (1 atm = 760 torr)
 = final volume = ?
= final volume = ?
 = initial volume = 64.0 mL = 0.0640 L (1 L = 1000 mL)
= initial volume = 64.0 mL = 0.0640 L (1 L = 1000 mL)
Now put all the given values in the above formula, we get:
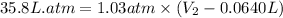
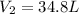
Thus, the final volume of the gas is, 34.8 L