Answer: The pH of the solution is 1.703
Step-by-step explanation:
We are given:
Concentration of monochloroacetic acid = 0.31 M
The chemical equation for the dissociation of monochloroacetic acid follows:
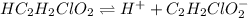
Initial: 0.31
At eqllm: 0.31-x x x
The expression of
 for above equation follows:
for above equation follows:
![K_a=\frac{[H^+][C_2H_2ClO_2^-}}{[HC_2H_2ClO_2]}](https://img.qammunity.org/2021/formulas/chemistry/high-school/v0mfqjxhdkp11nr4cotobwotzuh7k975t1.png)
We are given:
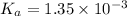
Putting values in above equation follows:
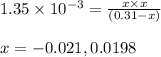
Neglecting the negative value of 'x' because concentration cannot be negative.
To calculate the pH of the solution, we use the equation:
![pH=-\log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/fi7xbn2q6p6sosuqayohrecmxrbau6j4s5.png)
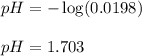
Hence, the pH of the solution is 1.703