Answer :
The value of standard Gibbs free energy is, 60.8 kJ
This reaction is reactant favored under standard conditions at 267 K.
Explanation :
As we know that,
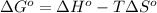
where,
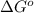 = standard Gibbs free energy = ?
= standard Gibbs free energy = ?
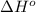 = standard enthalpy = 98.8 kJ = 98800 J
= standard enthalpy = 98.8 kJ = 98800 J
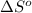 = standard entropy = 142.5 J/K
= standard entropy = 142.5 J/K
T = temperature of reaction = 267 K
Now put all the given values in the above formula, we get:
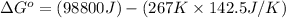
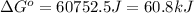
As we know that:
- A reaction to be spontaneous when
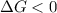 and reaction will be favored in the forward direction that means favored in products.
and reaction will be favored in the forward direction that means favored in products. - A reaction to be non-spontaneous when
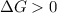 and reaction will be favored in the backward direction that means favored in reactants.
and reaction will be favored in the backward direction that means favored in reactants.
As, the value of
 is more than zero that means the reaction is non-spontaneous and reaction will be favored in the backward direction that means favored in reactants.
is more than zero that means the reaction is non-spontaneous and reaction will be favored in the backward direction that means favored in reactants.