Answer: Rate will increase by a factor of 2.
Step-by-step explanation:
As the reaction proceeds by an
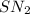 mechanism, both the reactants take part in the reaction and the rate law for the reaction will be:
mechanism, both the reactants take part in the reaction and the rate law for the reaction will be:
![Rate=k[NaN_3]^1[CH_3I]^1](https://img.qammunity.org/2021/formulas/chemistry/college/s38s0pcl23oc5dgmkqykqjcuh8lutkw0ot.png)
Thus when concentration of
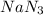 is doubled ,
is doubled ,
![Rate'=k[2NaN_3]^1[CH_3I]^1](https://img.qammunity.org/2021/formulas/chemistry/college/pc0yrtwitae3aj0lagashgg0b2jlsu1uze.png)
![Rate'=2* k[NaN_3]^1[CH_3I]^1](https://img.qammunity.org/2021/formulas/chemistry/college/pc2zts3muzedgf6sp42i5yuxyho0cbla4z.png)
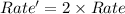
Thus the rate of the reaction would increase by a factor of 2.