Answer: The amount of aluminium reacted is 0.0324 grams
Step-by-step explanation:
We are given:
Vapor pressure of water = 26.8 mmHg
Total vapor pressure = 751 mmHg
Vapor pressure of hydrogen gas = Total vapor pressure - Vapor pressure of water = (751- 26.8) mmHg = 724.2 mmHg
To calculate the amount of hydrogen gas collected, we use the equation given by ideal gas which follows:
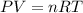
where,
P = pressure of the gas = 724.2 mmHg
V = Volume of the gas = 46.5 mL = 0.0465 L (Conversion factor: 1 L = 1000 mL)
T = Temperature of the gas =
![27^oC=[27+273]K=300K](https://img.qammunity.org/2021/formulas/chemistry/college/h77ylapf6n7yvd90kc8ykrt8ns8s0vkucn.png)
R = Gas constant =
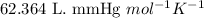
n = number of moles of hydrogen gas = ?
Putting values in above equation, we get:
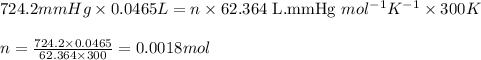
The chemical equation for the reaction of aluminium with HCl follows:
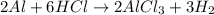
By Stoichiometry of the reaction:
3 moles of hydrogen gas is produced when 2 moles of aluminium is reacted
So, 0.0018 moles of hydrogen gas will be produces when =
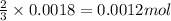 of aluminium is reacted
of aluminium is reacted
To calculate the mass from given number of moles, we use the equation:
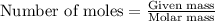
Moles of aluminium = 0.0012 moles
Molar mass of aluminium = 27 g/mol
Putting values in above equation, we get:
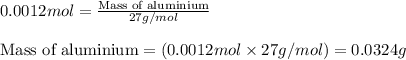
Hence, the amount of aluminium reacted is 0.0324 grams