Answer: The Gibbs free energy of the given reaction is -40 kJ
Step-by-step explanation:
The given chemical equation follows:
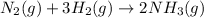
The equation for the standard Gibbs free change of the above reaction is:
![\Delta G^o_(rxn)=[(2* \Delta G^o_f_((NH_3(g))))]-[(1* \Delta G^o_f_((N_2(g))))+(3* \Delta G^o_f_((H_2(g))))]](https://img.qammunity.org/2021/formulas/chemistry/college/lenbux52c2ixv5no515ez2et6kkcc2w4pr.png)
We are given:
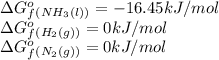
Putting values in above equation, we get:
![\Delta G^o_(rxn)=[(2* (-16.45))]-[(1* (0))+(3* (0))]\\\\\Delta G^o_(rxn)=-32.9kJ/mol](https://img.qammunity.org/2021/formulas/chemistry/college/pwvjtf1k6fw4ve8xxf7l2r126gyenpbjvz.png)
The equation used to Gibbs free energy of the reaction follows:
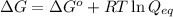
where,
 = free energy of the reaction
= free energy of the reaction
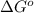 = standard Gibbs free energy = -32.9 kJ/mol = -32900 J/mol (Conversion factor: 1 kJ = 1000 J)
= standard Gibbs free energy = -32.9 kJ/mol = -32900 J/mol (Conversion factor: 1 kJ = 1000 J)
R = Gas constant = 8.314J/K mol
T = Temperature =
![25^oC=[273+25]K=298K](https://img.qammunity.org/2021/formulas/chemistry/college/xuv7m8qes7ftb60bgj8ln796iseb65owra.png)
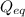 = Ratio of concentration of products and reactants at any time =
= Ratio of concentration of products and reactants at any time =
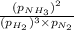
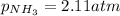
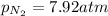
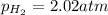
Putting values in above equation, we get:
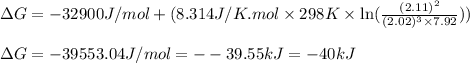
Hence, the Gibbs free energy of the given reaction is -40 kJ