Step-by-step explanation:
Formula for maximum efficiency of a Carnot refrigerator is as follows.
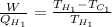 ..... (1)
..... (1)
And, formula for maximum efficiency of Carnot refrigerator is as follows.
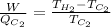 ...... (2)
...... (2)
Now, equating both equations (1) and (2) as follows.
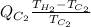 =
=
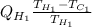
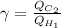
=
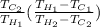
=
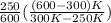
= 2.5
Thus, we can conclude that the ratio of heat extracted by the refrigerator ("cooling load") to the heat delivered to the engine ("heating load") is 2.5.